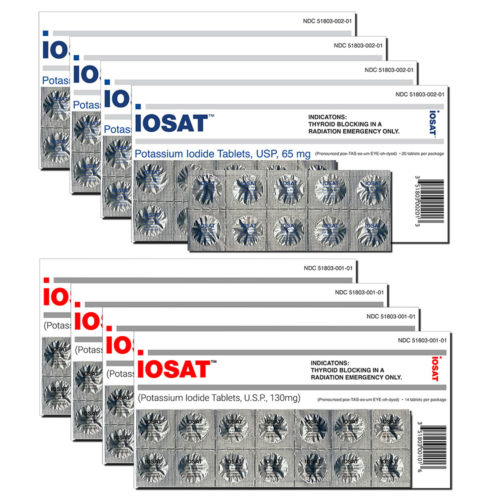
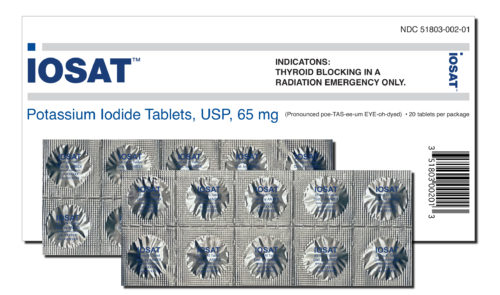
The only full-strength tablet approved by the FDA. For use by adults in a nuclear radiation emergency.
14 individually sealed 130mg tablets per pack.
Current Expiration Date: April 2032
Made in the U.S.A.
$19.95
In stock
The only full-strength tablet approved by the FDA. For use by adults in a nuclear radiation emergency.
14 individually sealed 130mg tablets per pack.
Current Expiration Date: April 2032
Made in the U.S.A.
In stock
Search the FDA Orange Book Database. There are only TWO brands of potassium iodide that can legally sold in the U.S. to prevent thyroid cancer from radiation exposure. EVERY Iosat™ batch is analyzed by the FDA for safety, quality, efficacy and 10-year shelf life sustainability.
Storing at least one pack (14 tablets) PER person for each location frequented (home, 2nd home, relative’s home, childcare, office, etc.) is our recommendation. Iosat Potassium Iodide tablets should be immediately available in the event of a nuclear radiation emergency. To be most effective, the first dose should be administered no later than 2-3 hours prior to potential exposure to radioactive iodine (as directed by public health officials). Administer one dose every day (same time) until your family has evacuated to an uncontaminated area, and then stop administering the tablets (as directed by public health officials). Any remaining tablets will still be foil-sealed and available for a future need.
| Weight | .5 oz |
|---|---|
| Current Expiration Date | April 2032 |
| NDC# | 51803-001-01 |